Building Medical Device Innovations Together
Tutorials & Reports
Sharing important insight among colleagues helps to drive the industry forward as a whole. That is why we feel it is important to create and share our tutorials, reports, and resources. See below for the latest from the Telos team.
Scientific data serves as the foundation of evidence-based medicine and is a key ingredient to successful medical device commercialization and sustainable growth.
Tutorial: Quantifying Probability of Benefit
When is a patient feeling better?
When is a patient feeling good?
What is the “probability of benefit” and how can we measure it for new technology?
Most often, clinical studies use validated patient-reported outcomes (PROs) to measure improvements in pain, function, or quality of life among the study participants. It’s important to select the right PROs for your technology, the disease or pathology being treated, and your research questions.
Check out the free tutorial from Jason Inzana, PhD, General Manager for Telos Partners LLC. →
Report: Evidence Development & Dissemination Strategy White Paper
Please complete the form below to access and download the PDF whitepaper:

A Comprehensive Approach to Generating and Leveraging the Currency of Scientific Evidence
As we know, scientific data serves as the foundation of evidence-based medicine and is a key ingredient to commercialization and growth.
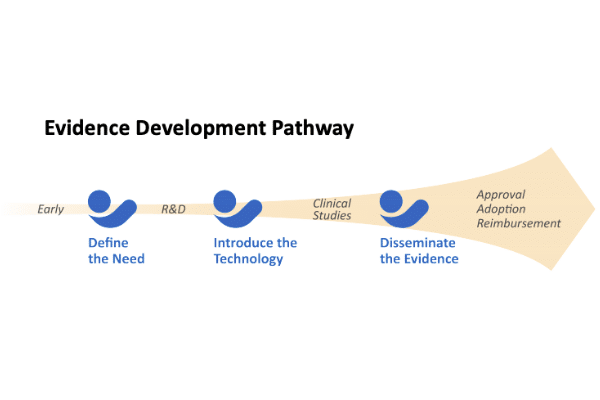
This white paper focuses on creating a plan for developing and disseminating the science necessary to support clinician adoption, regulatory approval/clearance, and reimbursement.
Key takeaways from the white paper:
-
Identifying the evidence development pathway
-
How to build a claims and objectives matrix
-
Generate a scorecard to assist in appraising the current evidence and ongoing studies
-
How to identify gaps in evidence
-
Get an example template to help map out timelines, investment, and types of studies/data analysis to help support claims and value propositions